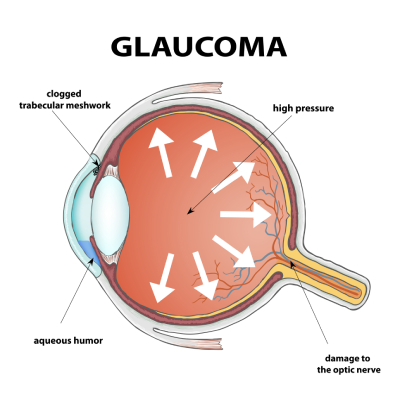
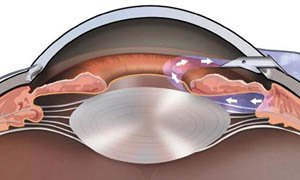
Glaucoma is a group of eye diseases in which an increase in the pressure inside the eye (intraocular pressure or IOP) results in damage to the optic nerve and leads to vision loss.
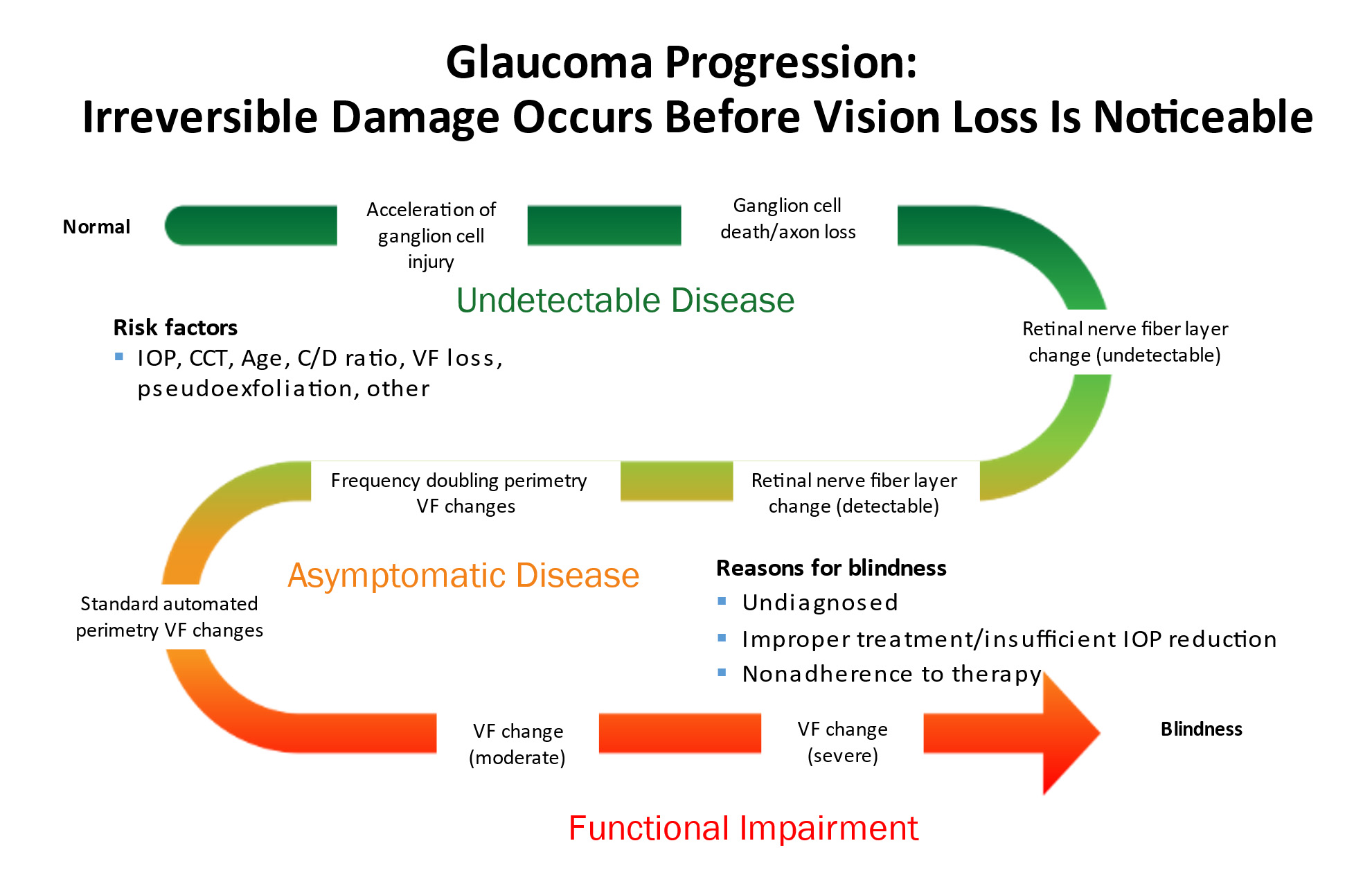
The vision loss from glaucoma can be mild and may not even be noticeable by the patient. However, sometimes it can progress to severe vision loss. Glaucoma can even lead to total blindness. The rate of disease progression can also vary significantly . Some patients progress very slowly over the course of many years. Others can develop very rapid vision loss in just a few weeks or months.
Glaucoma is often called the sneak thief of sight because by the time a patient develops symptoms of vision loss, there is already significant damage to the optic nerve.
Glaucoma is treated by lowering the intraocular pressure in order to stabilize the optic nerve and prevent the development or progression of vision loss. The treatments can be medical, glaucoma laser, or glaucoma surgery.
Vision loss in glaucoma can be prevented with early diagnosis and proper treatment.Compliance with treatment and regular follow-up examinations are central to glaucoma management.
The Eye and Vision
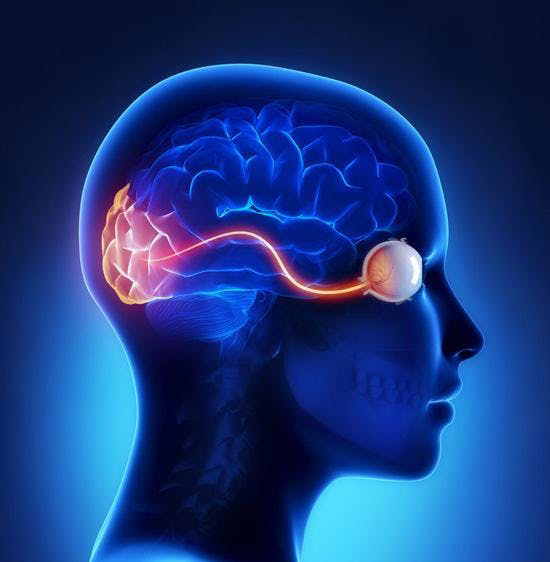
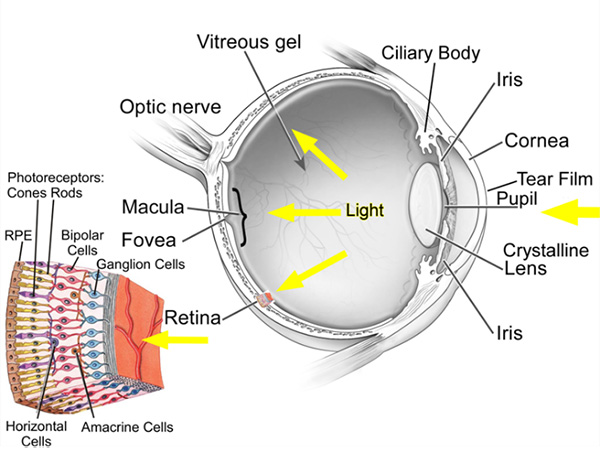
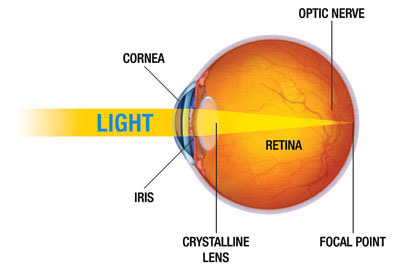
The eye is the organ of sight. It functions to focus incoming light energy, convert it to electro-chemical impulses, and send these impulses to the brain. The retina is the light sensitive film in the back of the eye. Inside specialized cells in the retina, which are called photoreceptors (the rods and cones), the incoming light causes chemical reactions which create the electro-chemical impulses. The photoreceptors are connected to other cells, called retinal ganglion cells. The retinal ganglion cells connect the retina to the brain. The fibers (axons) of the retinal ganglion cells make up the optic nerve in the back of the eye.
While it is the eye that receives light impulses, the actual site of vision is in the brain (specifically, the occipital cortex, located in the back of the skull). Therefore, the visual pathway refers to the entire path that the light stimulus travels, from the point of entry in the eye until it reaches the occipital cortex . Any disease that affects any part of the visual pathway can result in vision loss.
In America, the most common causes of permanent vision loss are macular degeneration , diabetic eye disease, and glaucoma. Glaucoma is the most common cause of preventable vision loss and blindness.
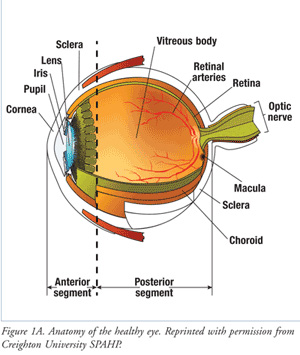
The Structure of the Eye
The eyeball is divided into front and back parts called the anterior segment and the posterior segment.
The anterior segment functions to focus the entering light, as it passes through the cornea and lens. The anterior segment also produces and drains the aqueous humor fluid.
The posterior segment is the larger portion of the eye and consists of the vitreous cavity, which contains the vitreous gel. The retina lines most of the posterior segment. The very back of the retina, where the incoming light is focused, is called the macula. The optic nerve leaves the back of the eye at the optic nerve head.
The full area that an eye sees, from the very central vison to the extreme side (peripheral) vision is referred to as the visual field. Vision loss can occur in any or all parts of the visual field. Certain patterns of visual field loss are characteristic of different diseases. The range of visual field loss can vary from being very mild without symptoms, to very severe causing blindness.
Intraocular Pressure (IOP)
The eyeball needs a proper amount of pressure (the intraocular pressure or IOP) to maintain its shape. The normal IOP is in the range of 10 to 21 mm Hg, with most people falling in the 16 to 20 range. (The unit of eye pressure is millimeters of mercury or mm Hg, the same units as blood pressure. However, eye pressure and blood pressure are unrelated.) As the IOP increases above the normal range, there is a corresponding increase in the risk of vision loss from glaucoma.
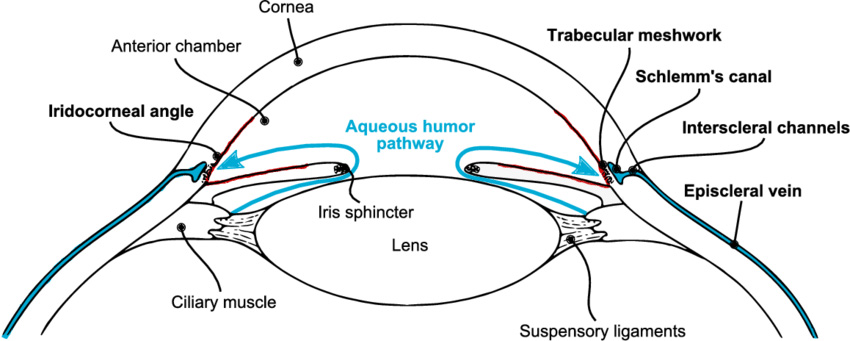
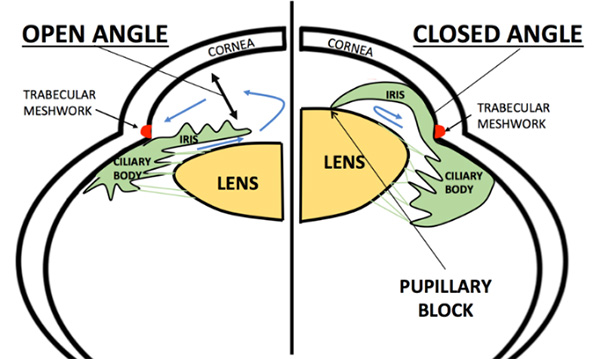
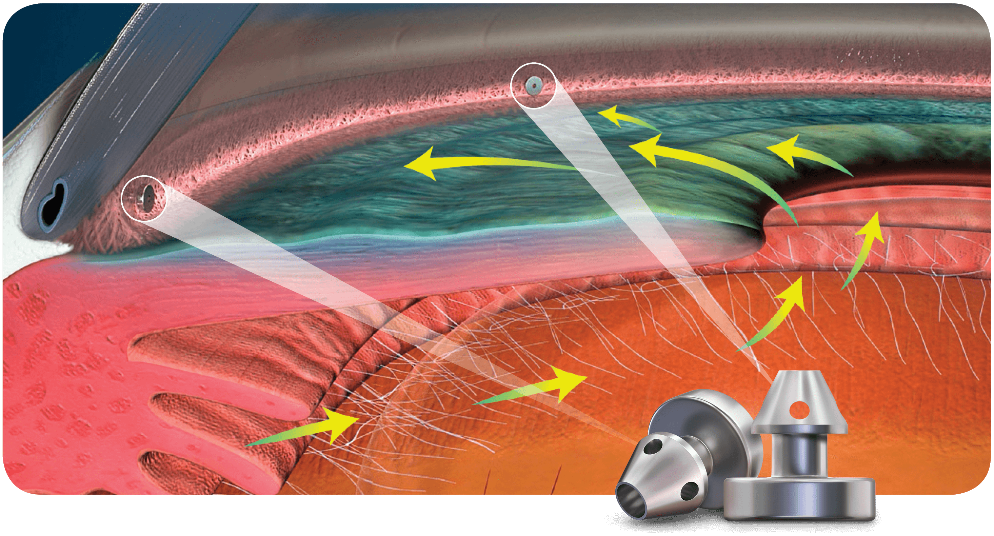
The eyeball continuously makes a clear fluid to maintain the eye pressure (called the aqueous humor or simply aqueous.) The aqueous is made by muscle tissue near the front of the eye called the ciliary body. The aqueous then flows through the pupil and filters out through the trabecular meshwork, eventually draining into the venous circulation.
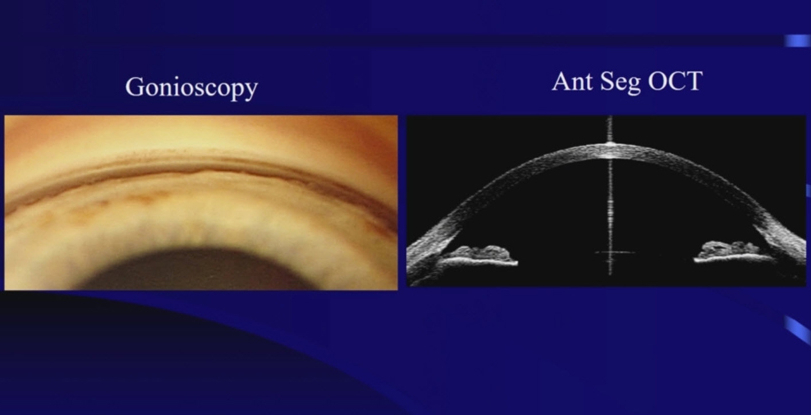
The area where the fluid leaves the eye through the trabecular meshwork is called the drainage angle (iridocorneal angle), because it is located at the base of an angle made by the cornea and iris. Analysis of the drainage angle is performed with gonioscopy.
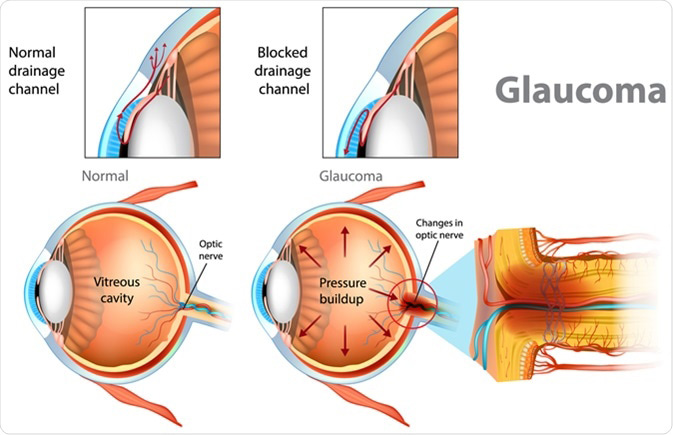
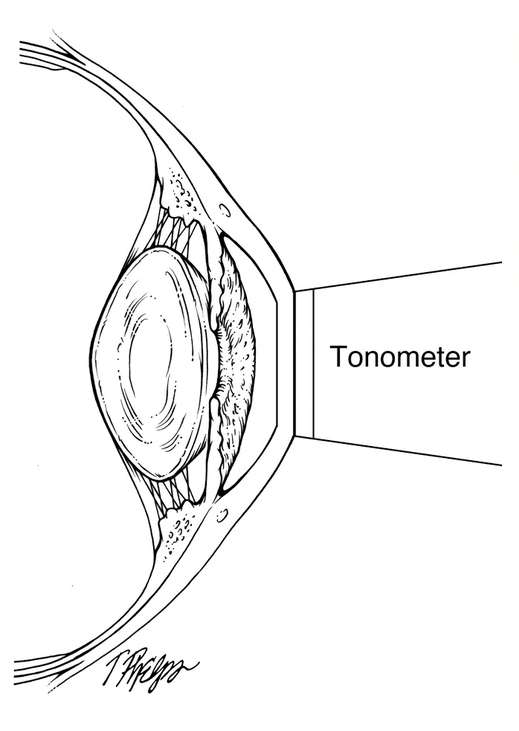
The IOP can become elevated for many different reasons. In general, when the IOP is too high it is because the aqueous does not drain properly from the eye. The IOP is measured by a test called tonometry.
Optic Nerve Damage in Glaucoma
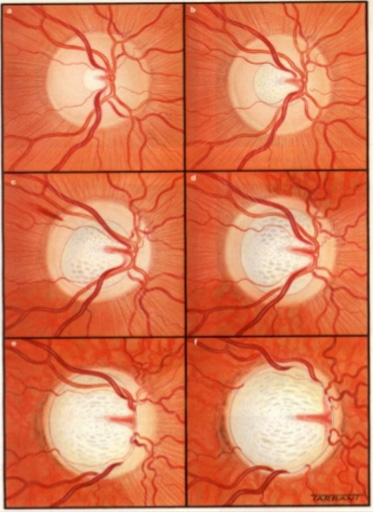
When the IOP becomes too high, it causes damage to the optic nerve head. Although the front of the eye is the region where the aqueous is made, the increased IOP causes damage to the optic nerve head in the back of the eye, because this is the “weak” spot of the eyeball. [The surrounding tissue of the eye (the cornea and sclera) is quite strong and resistant to high pressure compared to the optic nerve.]
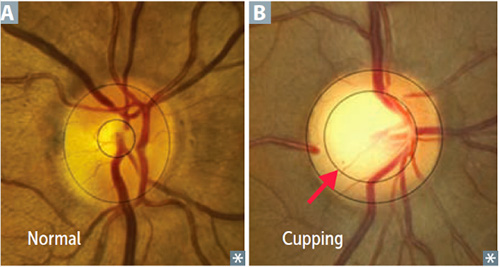
The change in the optic nerve head as a result of high IOP is called cupping. The optic nerve head changes shape with the central portion enlarging and bending backwards.
As the damage to the optic nerve continues, the eye will develop visual field loss. Certain patterns of visual field loss are characteristic of glaucoma and will continue to worsen if the disease progresses. Visual field testing is a fundamental part of glaucoma management.
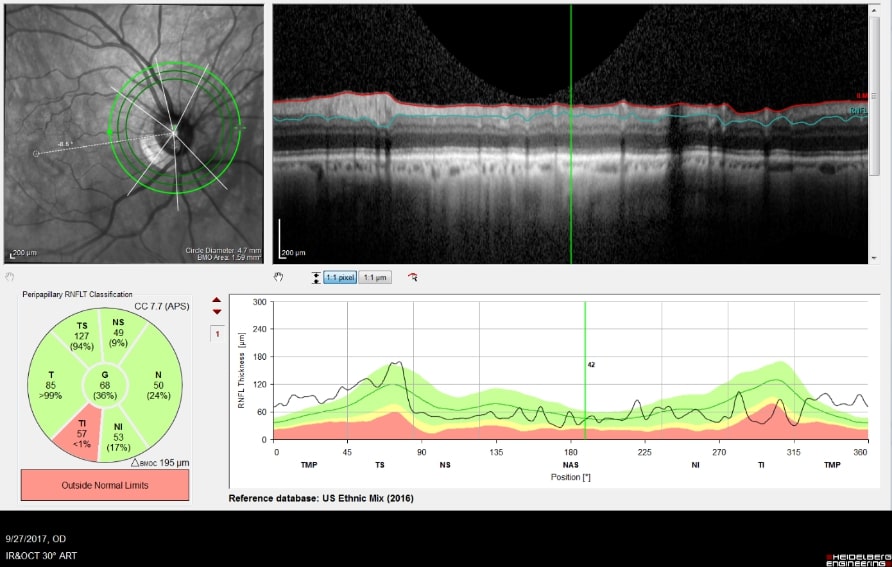
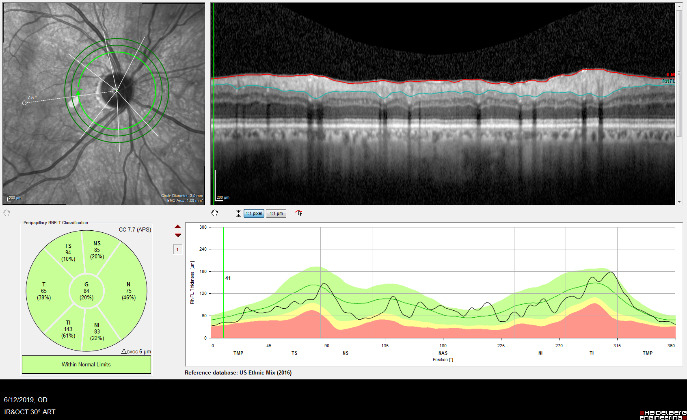
As the cupping increases, the axons of the optic nerve become damaged, causing the retinal ganglion cells to die. The fibers of axons make up the nerve fiber layer of the retina. As glaucoma worsens the nerve fiber layers thins. The thinning of the nerve fiber layer and cupping of the optic nerve head can be measured and monitored very precisely with specialized diagnostic testing, called OCT (Optical Coherence Tomography).
There is no feedback from the optic nerve head to the front of the eye. Therefore, optic nerve damage will continue unless treatment is initiated to lower the IOP. When vision loss develops in glaucoma it is permanent because the retinal ganglion cells do not regenerate and cannot be replaced.
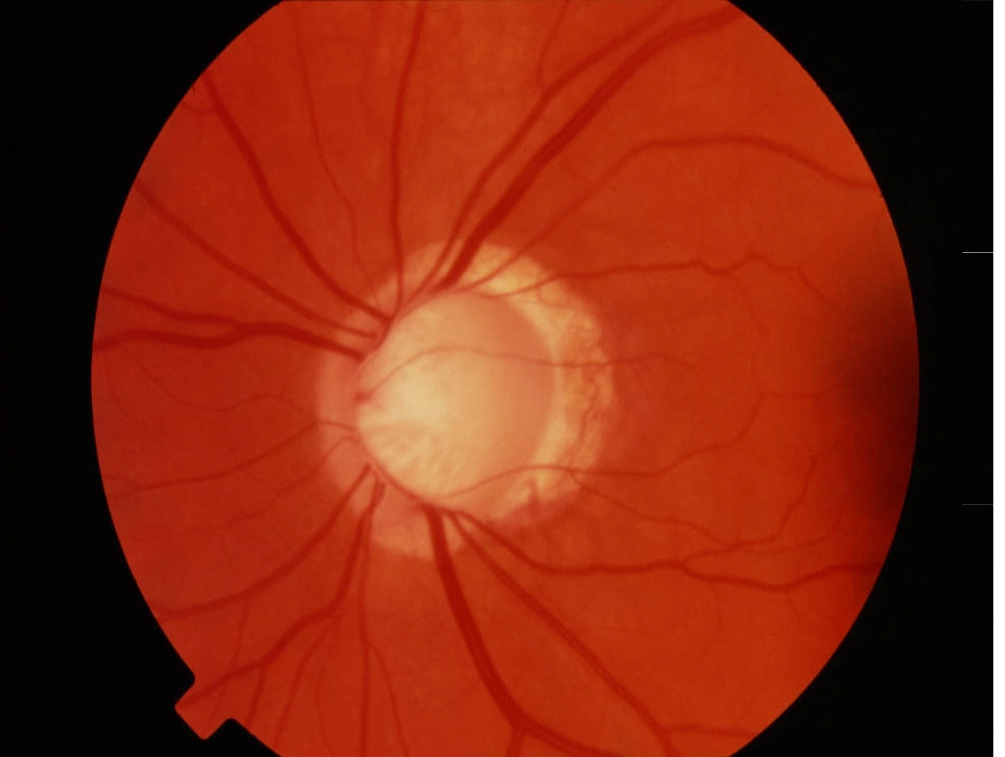
Optic Nerve Head appearance in a normal eye (A)
and an eye with glaucoma (B)
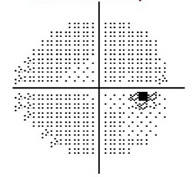
Normal Visual Field
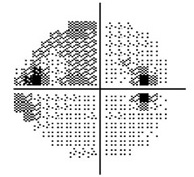
Moderate Visual Field Loss
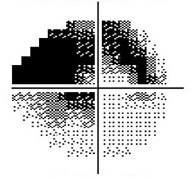
Severe Visual Field Loss
Types of Glaucoma
There are many different types of glaucoma. They all share in common the potential to cause vison loss by causing increased IOP and damaging the optic nerve. They differ in the underlying cause of the high IOP. Because most causes of increased IOP involve the front portion of the eye, they are referred to as anterior segment diseases. The types of glaucoma are divided into either primary glaucoma if they are due to an intrinsic defect in the eye, or secondary glaucoma if they are due to something that indirectly leads to increased IOP. Glaucoma is also divided into open angle and closed angle types. The major types of glaucoma are described here.
Primary Open Angle Glaucoma, or POAG,is the most common type of glaucoma. This glaucoma usually develops in adulthood. It is causes by a defect in the outflow channel, probably at the level of the trabecular meshwork, such that the aqueous fluid does not drain adequately, and the pressure builds up in the eye. The cause is due ti an intrinsic defect in the eye, and it is not related to any other disease or condition in the body.
Angle Closure Glaucoma results from a narrowing of the angle between the iris and cornea. Angle closure glaucoma can be gradual and chronic, or it can be sudden and acute. In cases of acute angle closure glaucoma, (sometimes called a glaucoma attack, the IOP can become dangerously high, casing severe pain and rapid loss of vision.
The angle can sometimes be narrow, but not yet fully closed, putting the eye at risk for an angle closure attack. Eyes that are far-sighted (hyperopic) are at increased risk for angle closure glaucoma.
The treatments for angle closure glaucoma include laser iridotomy or lens removal (either as cataract surgery or clear lens extraction).
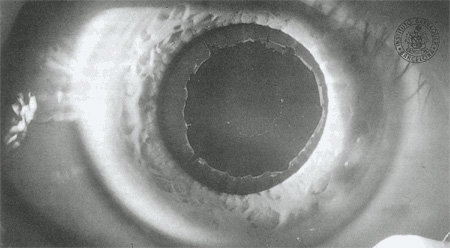
Exfoliation Glaucoma (also called pseudoexfoliation glaucoma or PXF) is a common form of a primary glaucoma where exfoliative (flaky or “dandruff like”) material is produced in the anterior segment and blocks the drainage angle, causing elevated IOP. Exfoliation glaucoma can be aggressive, causing exceedingly high eye pressures. Often, it affects just one eye first, then months to years later, can affect the fellow eye. Exfoliating glaucoma is common in people with lighter colored eyes and people of Northern European ancestry. Exfoliation glaucoma often makes cataract surgery more complex.
Exfoliation material seen on the surface of the lens, behind the pupil.
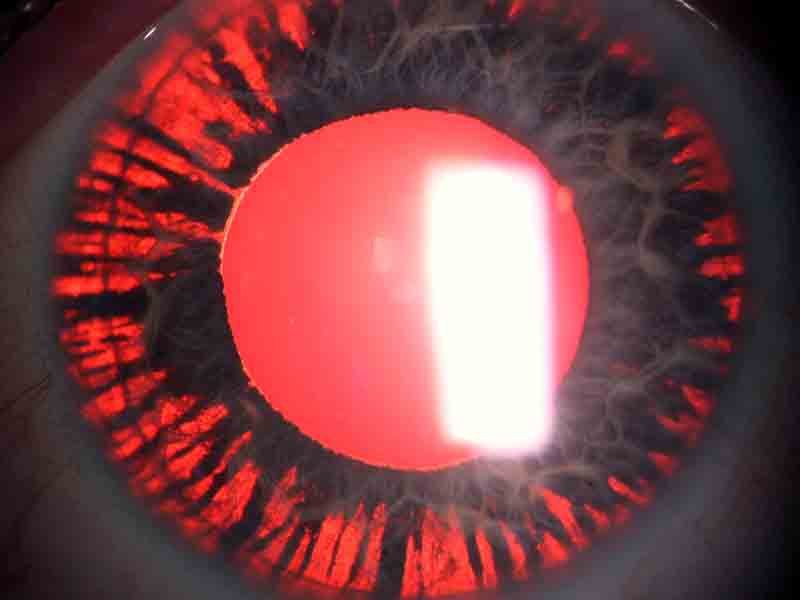
Pigmentary glaucoma (PG) is an aggressive form of glaucoma that is often seen in younger people, and those who are near-sighted (myopic). It can develop when patients are in their teens or 20s. It frequently goes undetected until the disease is advanced, since it can require a detailed examination to accurately diagnose, including gonioscopy. Pigmentary glaucoma is caused by a chaffing of the iris by the lens zonules, with the dispersed pigment then clogging the trabecular meshwork. Eventually, enough pigment can be released so that light passes directly though the iris, an effect referred to as transillumination defects.
An eye with pigmentary glaucoma demonstrating transillumination defects.
Steroid-induced glaucoma is a common type of secondary glaucoma and can result from the use of many types of steroids, whether taken orally, in inhalers, eye drops, ointments, or creams. The eye pressure can become extremely high after just a few weeks of steroid use. Conditions where steroids are commonly used as treatment include COPD (asthma, emphysema, or chronic bronchitis), arthritis (especially rheumatoid arthritis), inflammatory bowel disease, and others. Steroids that cause glaucoma are also a common cause of cataracts.
Glaucoma is an eye disease in which the eye pressure (intraocular pressure) is too high for the eye, causing damage to the optic nerve, resulting in loss of vision.
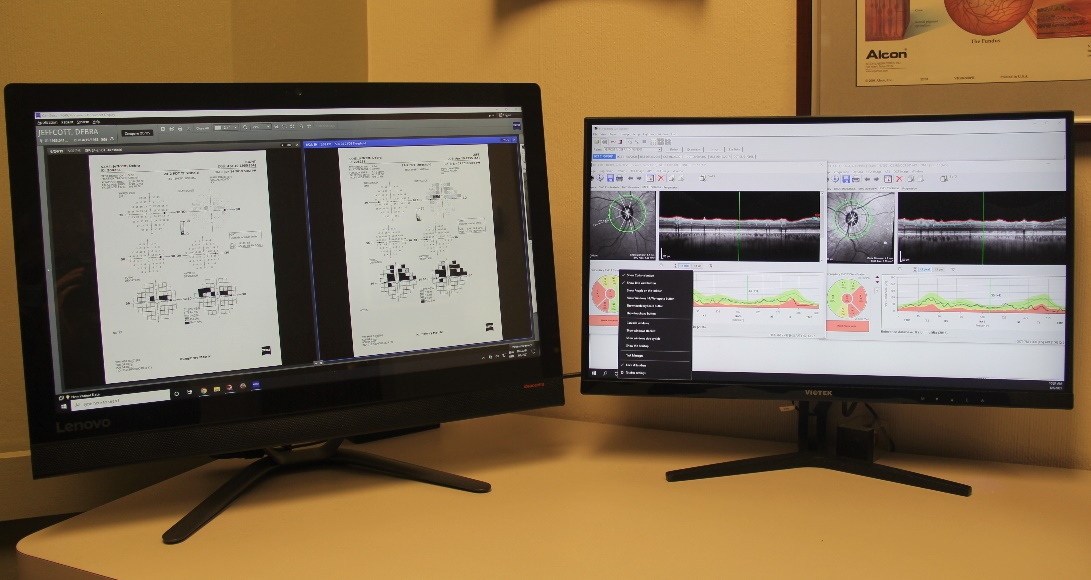
Proper glaucoma management involves examining and monitoring all parts of the eye that are related to the pressure and optic nerve function. This is accomplished by employing a variety of testing modalities. Not every test needs to be performed at each examination. But it is important that the appropriate tests are performed when needed.
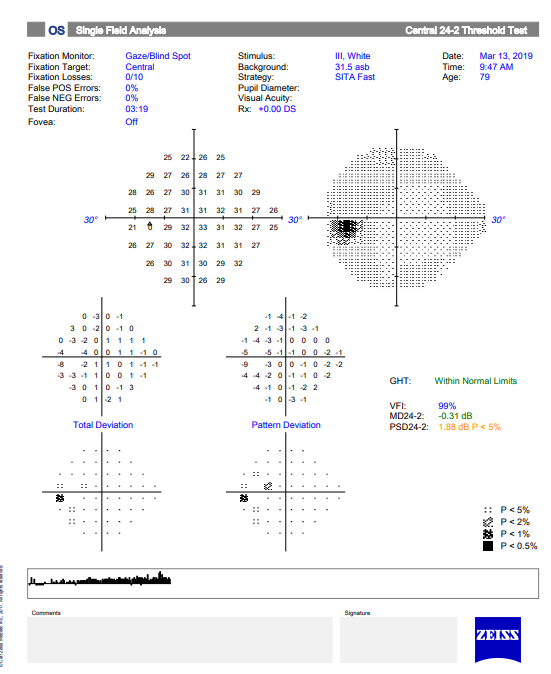
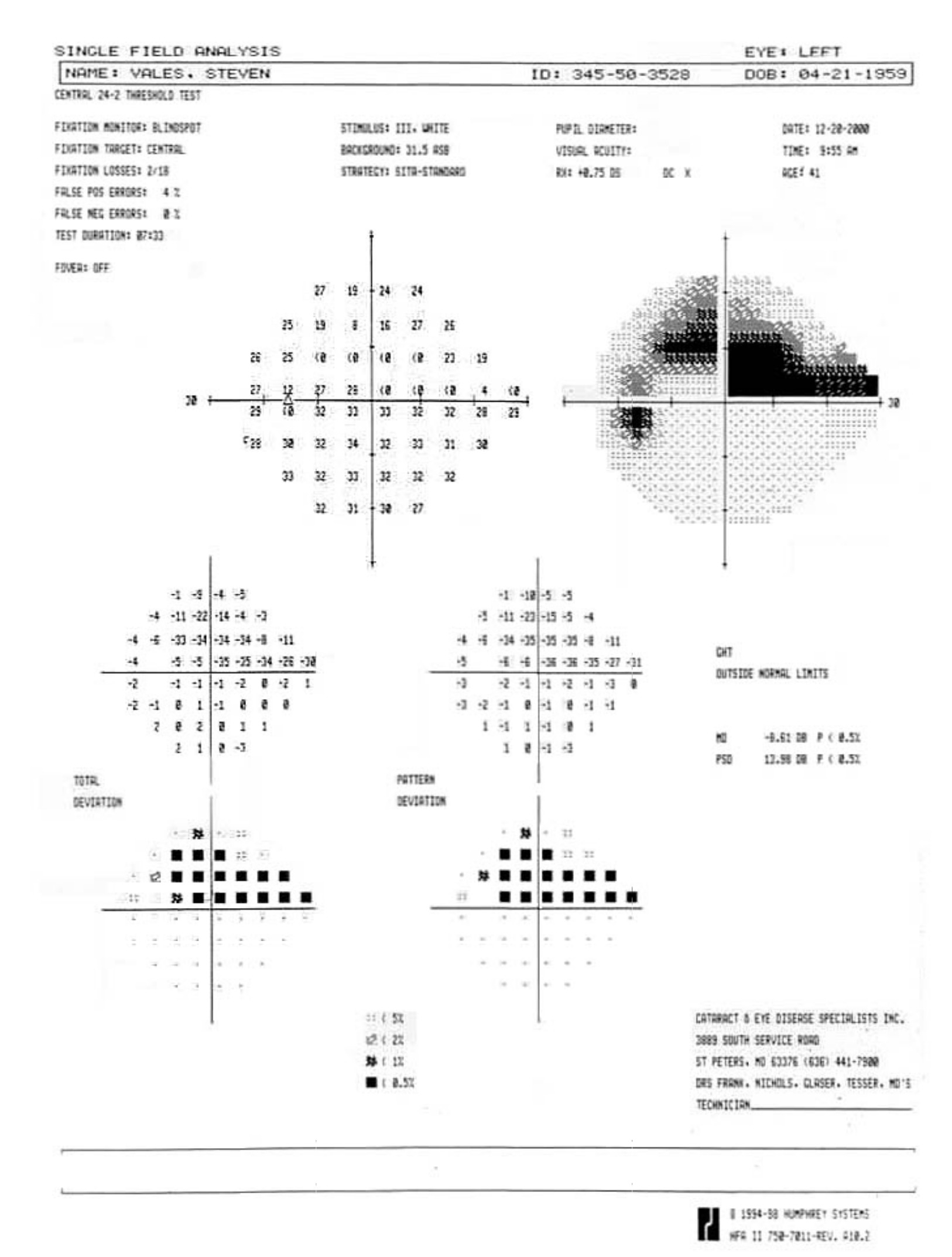
Visual Field Testing is an essential component of glaucoma diagnosis and management.
The visual field refers to the entire area that an eye sees, from the outside (peripheral) vision to the very central vision. When the optic nerve is damaged in glaucoma, this results in loss of parts or all the visual field. The lost area of visual field is referred to as a blind spot or scotoma. The main goals of glaucoma therapy are to prevent the development of visual field loss, and if visual field loss has already developed, to prevent further progression.
Often, the visual field loss is not noticeable by the patient until it is advanced, at which point it can interfere with regular activities such as driving:
Normal Visual Field
Advanced Visual Field Loss
Severe Visual Field Loss
The device used to test the visual field is called a perimeter, and visual field testing is also called perimetry. There are several types of perimeters available, and each has different types of test patterns and stimuli. The testing involves the patient looking into the perimeter bowl, while focusing (fixating) on a central point. Different types of target light (the stimulus) are then projected at random points in the perimeter and the patient clicks a button when a target light is seen. The visual field is then mapped out and displayed.
Visual field testing is a simple test and usually takes just a few minutes to perform on an eye. Because of the central role that visual field testing plays in glaucoma management, it is important that patients undergo the appropriate type of testing at regular intervals, usually every few months, in order to ensure accuracy.
The two main visual field tests that we perform on patients are Standard Automated Perimetry (SAP) and Frequency-Doubling Perimetry (FDP). SAP functions better to monitor disease progression and FDP is more useful for early disease detection.
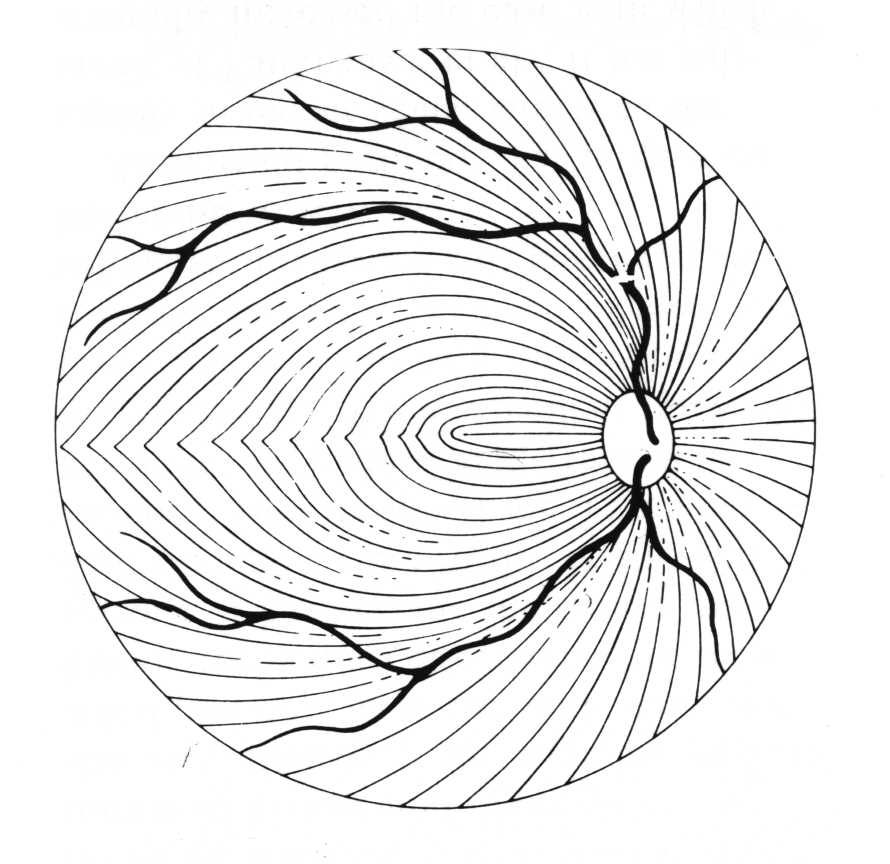
Because of the specific way the optic nerve and nerve fiber layer are damaged in glaucoma, the visual field loss that develops often appears in certain patterns. The nerve fiber layer extends across the retina in a curved pattern, so when it is damaged, the loss of vision follows that same pattern. These are usually called arcuate defects (since they are arc shaped).
Nerve Fiber Layer
Nerve Fiber Layer Damage
Arcuate Visual Field Loss
[Remember that the eye inverts an incoming image, so damage to the inferior (lower) nerve fiber layer results in superior (upper) visual field loss.]
In early glaucoma, the arcuate defects are small, and away from the central vision, so they might not be noticeable or symptomatic. If glaucoma worsens, the arcuate defects can extend from the peripheral to the central vision, at which point they start to affect how a patient sees. By the time a patient notices the visual field loss, the disease is considered advanced or severe.
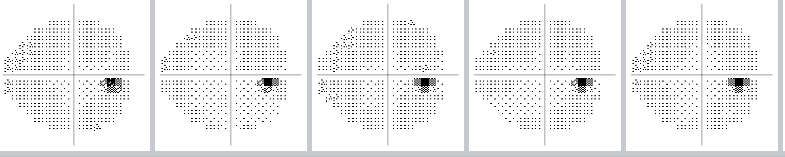
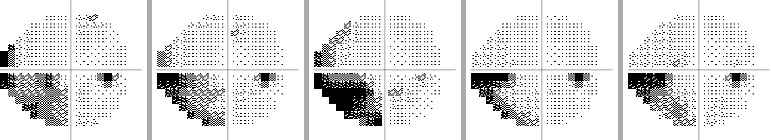
The visual field can be monitored over time to see if there is progression of glaucoma, or if the disease has stabilized as a result of treatment:
Example of a normal visual field over time
(The small dark spot is the eye’s "natural" blind spot.)
Example of a stabilized visual field over time
Example of progression of visual field loss over time
Optic Nerve Analysis is a central part of glaucoma diagnosis. Because the optic nerve is the site of injury in glaucoma, decisions about treatment are made based upon the degree of damage to the nerve.
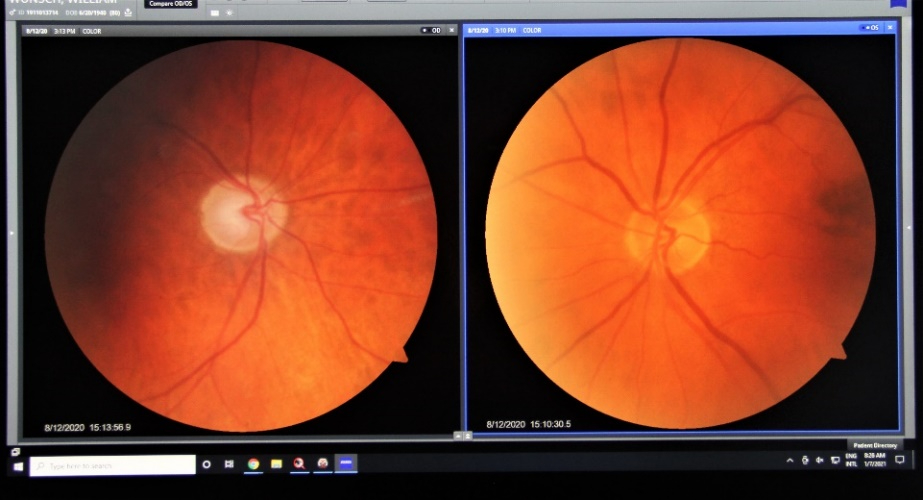
The optic nerve head is the part of the optic nerve that is susceptible to injury from elevated intraocular pressure. The optic nerve head can analyzed in three-dimensions by optical coherence tomography or imaged by fundus photography.
Optical Coherence Tomography
Fundus Photography
Each type of optic nerve analysis gives a different type of information Optical coherence tomography creates a 3-D image of the optic nerve head which can be monitored over time to see if there is are any changes in the nerve structure. . Fundus photography documents the appearance of the nerve in real color and allows for comparison with other examinations. As is the case for visual field testing, optic nerve analysis needs to be performed at regular intervals to determine if the glaucoma therapy is effective.
Optical Coherence Tomography (OCT) is an advanced imaging technology that has revolutionized the diagnosis and management of eye disease, especially glaucoma and retinal disease. OCT is a non-invasive method of imaging structures in the eye at an extremely high, microscopic resolution.
OCT uses infrared light to create cross-sectional, three-dimensional images of the tissues in the eye. As is the case for laser treatments, the eye is particularly amenable to imaging with OCT because the eye is optically transparent.
OCT Imaging of the Nerve Fiber Layer
OCT Image of the Retina
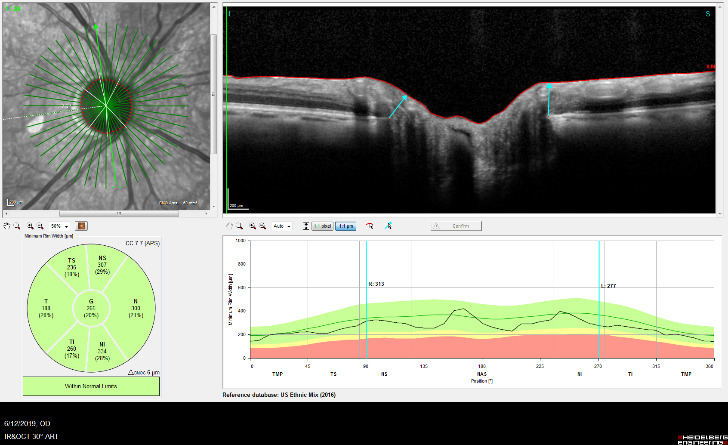
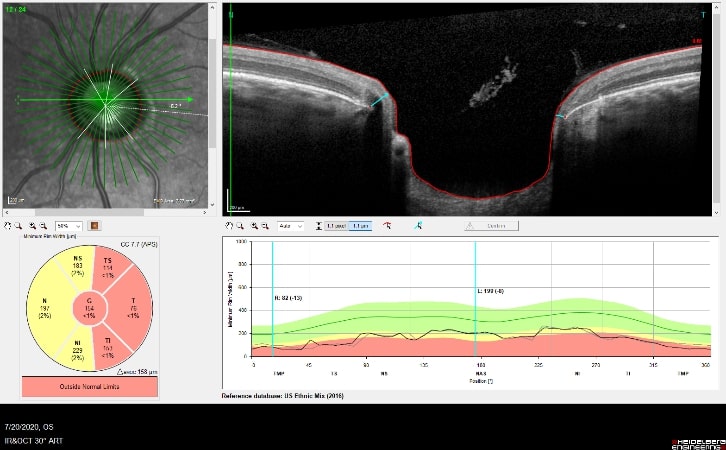
OCT is used in glaucoma to image the optic nerve and the nerve fiber layer in order to determine if there is damage from increased eye pressure. Because the resolution of OCT is so high, it can detect fine changes to the shape of optic nerve. Thinning of the nerve fiber layer is a sign that glaucoma is worsening and that the eye pressure is too high.
OCT Imaging of a Normal Optic Nerve
OCT Imaging showing a
Cupped Optic Nerve
Structural changes to the optic nerve or nerve fiber layer usually precede visual field loss. In many cases, 50% of the optic nerve can be damaged before visual field loss develops. As a result, OCT is a critical tool for diagnosing glaucoma at its earliest stages.
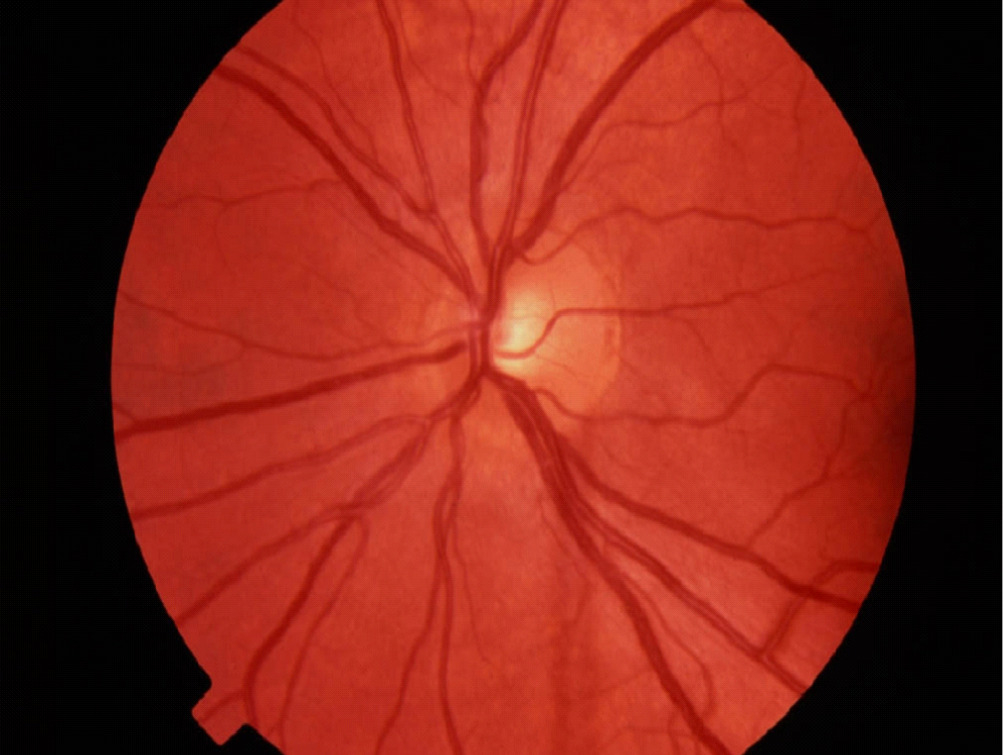
Fundus photography records digital photographic images of the back of the eye (the fundus). Specific parts of the fundus can be photographed such as the optic nerve and macula. Fundus photography compliments OCT in that it provides true color images of the different structures.
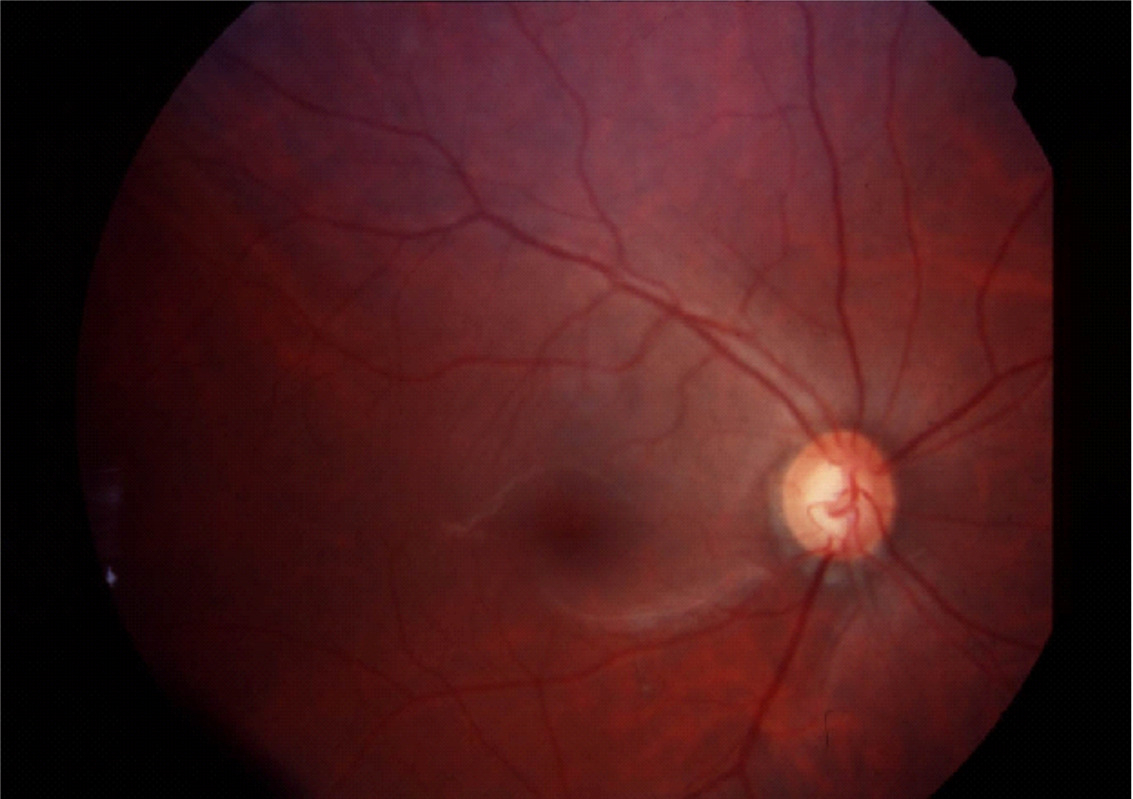
Normal Optic Nerve
Glaucomatous Cupping
Fundus photography allows documentation of changes to the optic nerve that occur glaucoma including cupping, disc hemorrhages, atrophy, and loss of the nerve fiber layer. Certain filters can be used to enhance the visualization the nerve fiber layer.
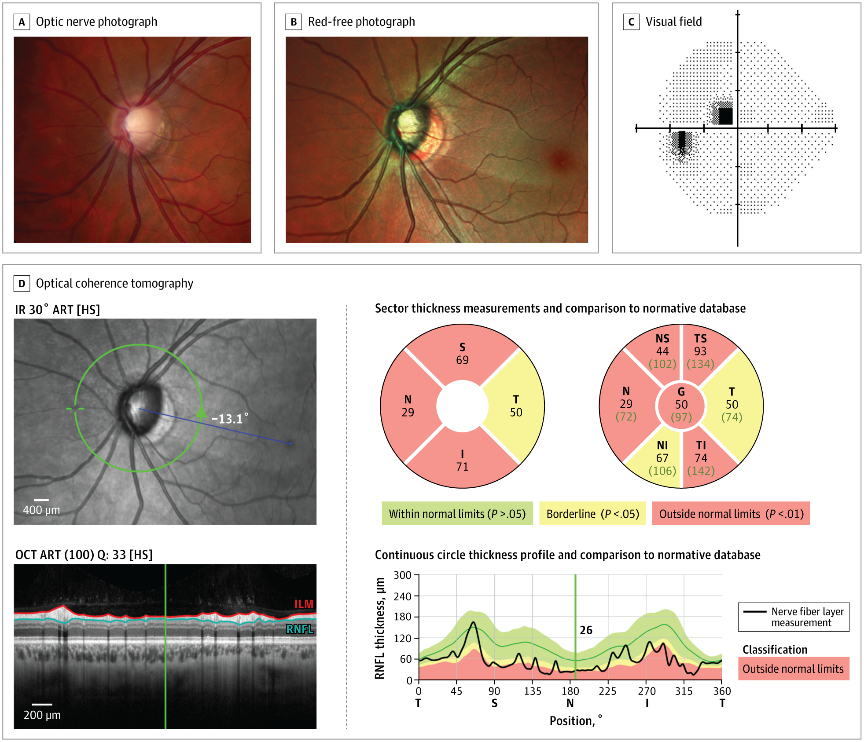
An example of an optic nerve with glaucoma damage imaged with both fundus photography and OCT and compared to the visual field in order to demonstrate the strucure-function relationship.
The lower half of the optc nerve has early damage, and this correlates with the small superior scotoma seen on the visual field test.
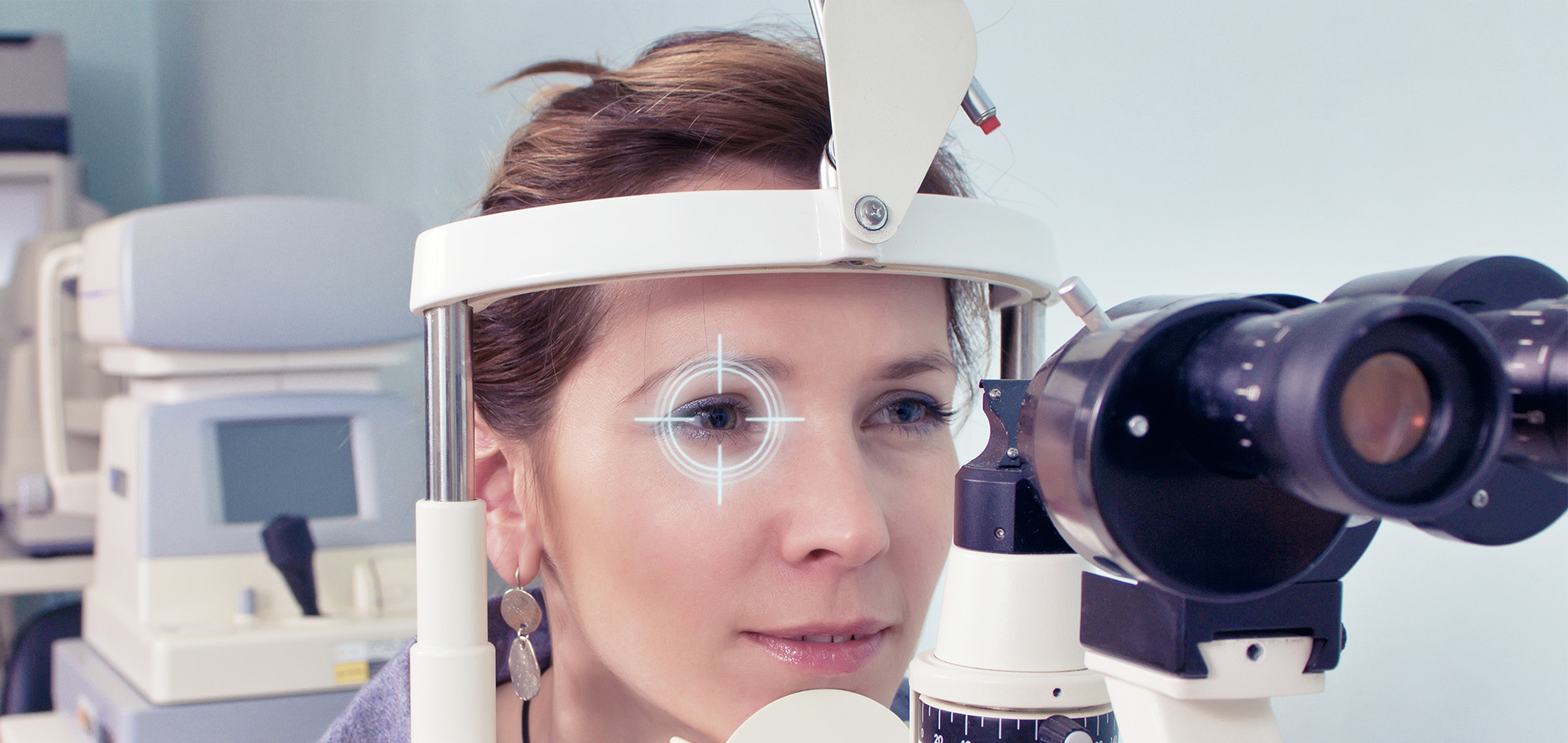
Intraocular Pressure (IOP) measurement is performed at every eye examination. Because all glaucoma treatments are designed to lower intraocular pressure, it is essential that accurate IOP measurements are made to determine effectiveness of treatment.
There are many different methods for measuring eye pressure. The most precise and reliable is Goldmann Applanation Tonometry (GAT, or simply applanation) . Other, less reliable methods include Non-Contact Tonometry (NCT, which is also referred to as the “air-puff” test) and the Tono-Pen (which is an electronic measurement).
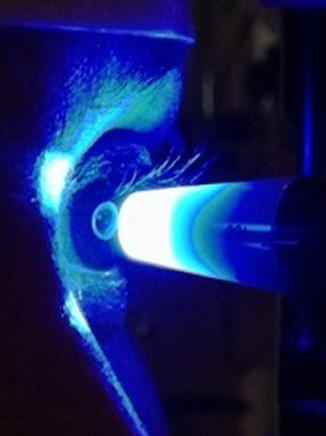
GAT determines the IOP by using a tonometer tip to physically contact the eye and flatten (applanate) the central cornea. The in order to visualize the applanation, an orange dye (fluorescein) is placed on the eye to color the tear film, along with a numbing drop. The tonometer tip is then illuminated with a blue light causing the dye to fluoresce. When the cornea is flattened with a pressure that equals the pressure in the eye, two semicircle rings line up as seen through the slit-lamp. The tension is then read off a dial on the tonometer base and converted to mm Hg (millimeters of Mercury, the units of pressure).
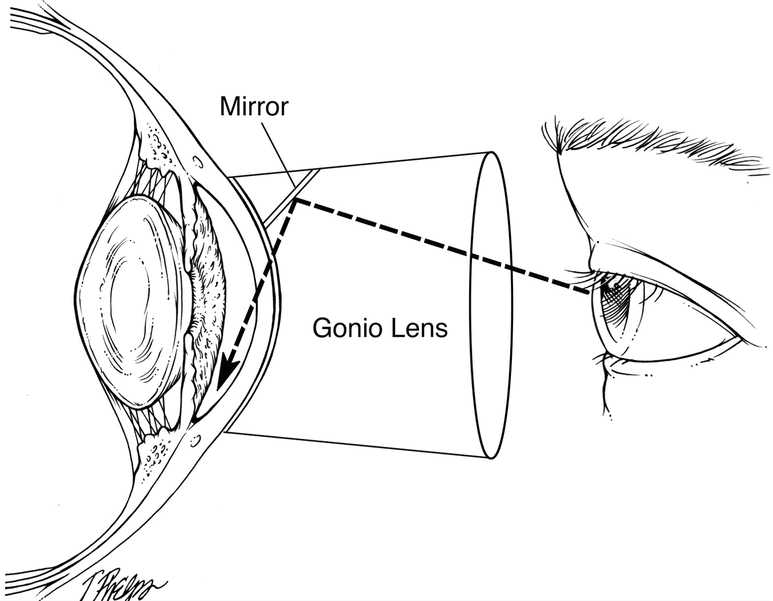
Gonioscopy examines the outflow angle where the aqueous humor drains from the eye. Gonioscopy determines whether the glaucoma is of the open angle or closed angle type.
Because of the structure of the eye, the outflow angle cannot be seen directly. A special lens, called a gonioprism is required to see the angle structures indirectly using reflected light. Because the angle is the site of the eye where Selective Laser Trabeculoplasty is performed, a gonioprism is also used for the laser treatment.
Optical Coherence Topography can also be used to analyze the anatomy of the outflow angle, in a three-dimensional view.
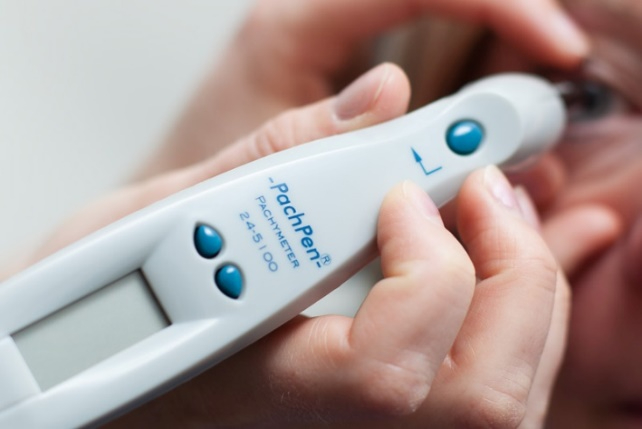
Pachymetry is a test that is performed to determine the central thickness of the cornea. Thinner corneas are associated with increased risk of glaucoma and thicker corneas are associated with a lower risk of glaucoma. The cornea itself is not involved in the development of glaucoma, but rather the thickness serves as a marker for relative risk.
Corneal pachymetry measurements are very quick to perform, using a hand-held electronic ultrasound device.
The only treatment that has been shown to benefit eyes with glaucoma is pressure reduction. Medical therapies have been a key component of glaucoma care for years. Based on the type of glaucoma and amount of pressure reduction required, there are multiple medication options for a patient with glaucoma. These include topical, oral, and injected medications. All of these treatments work to reduce the pressure in the eye and stabilize any optic nerve damage.
The most common form of medical treatment for glaucoma is topical eye drops. These drops are used on a daily basis. Most current medications are used either once or twice per day.
There are multiple types of glaucoma drops that can be divided into drug classes.
Prostaglandin Analogs
Beta Blockers
Carbonic anhydrase inhibitors
Alpha-agonist
Combination medications
Prostaglandin Analogs (PGAs) are often the first type of drop used when a patient is diagnosed with glaucoma. These drops are used once a day at bedtime. Prostaglandin Analogs lower the pressure by increasing the fluid outflow of the eye. Medications such as Lumigan, Travatan, Xalatan (latanoprost), and Zioptan are prostaglandin analogs. Recently medications that combine traditional prostaglandin analogs with other pressure lowering pathways have been FDA approved. Vyzulta combines latanoprost with nitric oxide and Rocklatan combines latanoprost with netarsudil to lower eye pressure. Prostaglandin analogs are well tolerated although pigment changes of the eyes and eyelash growth are noted by some patients.
Beta blocker medications such as Timoptic (timolol), Betagan, and Betimol work to lower eye pressure by decreasing fluid production in the eye. They do this by blocking beta adrenergic receptors. These drops are normally prescribed once or twice daily. While beta blockers are tolerated by most patients, they can increase breathing issues for people suffering from disorders such as COPD or asthma.
Carbonic Anhydrase inhibitors (CAIs) are a common class of glaucoma drops that inhibit the enzyme family carbonic anhydrase. This action interferes with the sodium pump of the ciliary body and decreases aqueous humor production lowering eye pressure. They are available as the eye drops Azopt (brimzolamide) and Trusopt (dorzolamide). CAI oral medications such as Diamox (acetazolamide) are also available although patients have more side effects on oral verses topical medications. CAIs are a sulfonamide and are not recommended for patients with a sulfa allergy.
Alpha-agonist medications stimulate alpha adrenergic receptors in the eye. They work by decreasing fluid inflow to the eye while stimulating increased outflow. The most common alpha-agonist used in glaucoma treatment is Alphagan (brimonidine). Alphagan in normally use two to three times daily.
Many topical glaucoma medications have been shown to be more effective when used in combination with another topical medication. Not only does this allow for lower eye pressures but it also is more convenient for patients by decreasing the number of eye drops they have to use each day. Common combination drops include Cosopt (dorzolamide/timolol), Combigan (brimonidine/timolol) and Simbrinza (brinzolamide/brimonidine).
Recently the first dissolvable implant for the treatment of glaucoma was approved by the FDA. Durysta (bimatoprost implant) is a dissolvable implant that is placed in the eye and slowly releases medicine over the next several months. The implant is very small and is placed into the eye during an office visit and then remains inside the eye until is completely dissolves. This is especially useful for patients that have difficultly using traditional eye drops.
LASERS (Light Amplification by Stimulated Emission of Radiation) are devices that use concentrated light energy for a large variety of purposes in research, industry, and medicine.
Medical lasers are used to treat tissues in the body usually by heating, cutting, or reshaping the tissue.
Lasers have a wide variety of applications in the eye. The eye is particularly amenable to the benefits of laser treatment because it is optically transparent. As a result, laser treatment can reach most parts of the eyeball.
Lasers can be used to treat glaucoma, cataracts, refractive error, corneal and retinal diseases.
[Laser vision correction, also called LASIK, treats refractive error (nearsightedness, far-sightedness, or astigmatism) is one of many laser applications for the eye.]
Laser treatments are named for the tissue that is being treated, the type of laser used, the effect on the tissue, or a combination of these.
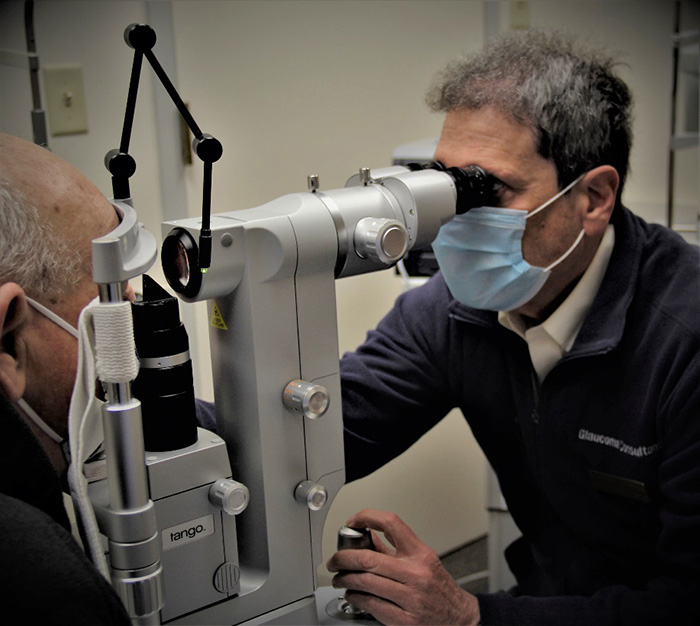
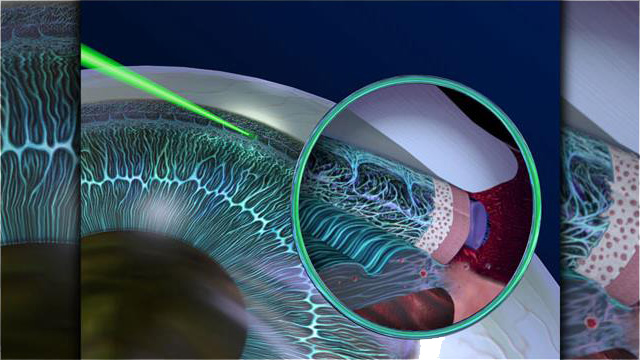
Selective Laser Trabeculoplasty (SLT) is an office-based laser treatment designed to lower the intraocular pressure (IOP). It is a simple and rapid procedure that is performed at the slit-lamp with minimal or no discomfort. The recovery is quick, and patients can resume regular activities immediately after the laser. The typical treatment time is just minutes per eye.
SLT treats the part of the eye called the trabecular meshwork, causing increased aqueous outflow, thereby lowering the IOP. It is often referred to as a “cold laser,” because it uses a mechanical pulse, rather than heat energy, to alter the trabecular meshwork.
SLT is successful approximately 80% of the time in lowering the IOP. For many patients, SLT can reduce or eliminate the need for glaucoma eye drops.
SLT can be repeated if the IOP lowering effect wears off. Generally, the IOP lowering effect can last three to five years or longer. In some patients, the IOP lowering effect is permanent after just one laser treatment.
There is little risk to undergoing SLT. There is no risk of infection and virtually no risk of bleeding. The main risks of SLT include occasional post-laser inflammation or a paradoxical IOP rise. These are usually transient side effects and resolve spontaneously or may require the temporary use of anti-inflammatory or glaucoma drops.
The IOP lowering effect takes a few weeks to occur, so patients are seen 2 to 3 weeks after the laser treatment to remeasure the IOP
In a small group of patients, there is no IOP lowering effect or the effect wears off rapidly in a few months. If this happens, it does not prevent the eye from undergoing other types of glaucoma treatment.
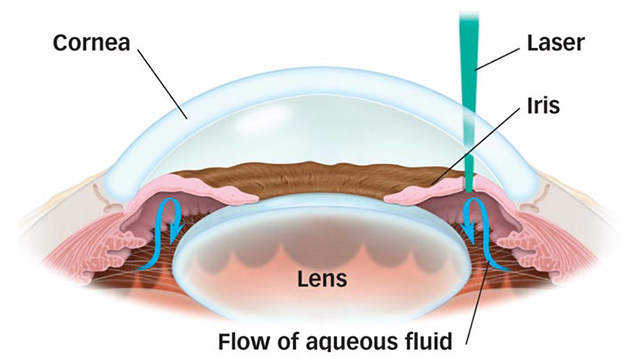
Laser Peripheral Iridotomy (LPI) is an office-based laser treatment designed to treat angle closure glaucoma and narrow angle glaucoma. The laser used, referred to as a YAG laser (Yttrium-Aluminum-Garnet), fires a brief, high energy laser pulse to create a tiny hole in the iris. This allows the aqueous to bypass the normal outflow path, which is through the pupil. As a result, the angle will open.
In cases where the angle is narrow, but not yet closed, the iridotomy will prevent frank angle-closure glaucoma attacks.
The treatment is performed rapidly at the slit-lamp with minimal or no discomfort. Patients can resume regular activities right after the laser treatment.
Complications of LPI are rare but can include bleeding, paradoxical pressure rise, or inflammation. Such post-laser problems will resolve spontaneously or with the use of eye drop medications.
LPI usually needs to be performed only one time and the iridotomy is permanent. Rarely, the iridotomy can close, requiring a supplemental treatment.
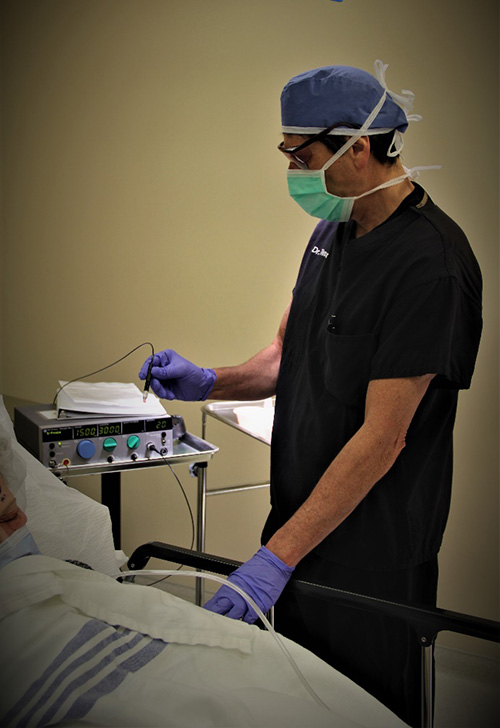
Diode Laser Transscleral Cyclophotocoagulation (Diode Laser or Diode TS-CPC) is a specialized type of glaucoma laser treatment. This laser treatment uses higher energy laser power to heat (photocoagulate) the ciliary body which is the part of the eye that produces the aqueous fluid. The laser probe physically touches the eye, and the laser energy is transmitted through the sclera to the ciliary body. As a result, the amount of aqueous produced by the ciliary body decreases, and this lowers the IOP.
Diode laser treatment is performed in the operating room so the patient can be sedated, and the eyeball numbed, to avoid treatment pain. The laser treatment itself is quick, and the treatment only take a few minutes.
Diode laser is usually reserved for glaucoma that is more difficult to control, or for patients where the vision is already significate compromised. Patients can resume regular activities the day after diode treatment. The IOP lowering effect is not immediate, so patients are examined usually one week after treatment.
There are several potential diode laser complications that can occur after treatment. There can be significant post-laser pain that can last for several days, requiring the use of pain medication. There can be significant inflammation, more than seen with office-based laser treatments, requiring the use of steroid or non-steroidal eye drops. The inflammation can cause swelling in the eye, included swelling of the retina. Long-term, the diode laser treatment can cause the cornea of the eye to become cloudy.
The pressure lowering effect from diode laser is often unpredictable. Sometimes the eye pressure can become too low. The pressure -lowering effect can also wear off suddenly after a short time, requiring an additional treatment. Occasionally, there is no effect.
Although the diode laser treatment has a higher side-effect profile that other lasers, and is more unpredictable, in many cases it is preferred to conventional glaucoma surgery because is very quick to perform, there is no risk of bleeding or infection, and there is a quick recovery.
Minimally Invasive Glaucoma Surgery (MIGS) refers to a group of procedures that are performed relatively quickly with little manipulation to the eye, usually under topical (eye drop) anesthesia, using small incisions and no sutures. The goal of a MIGS procedure is to provide rapid recovery while minimizing the risks associated with conventional glaucoma surgery.
As a general rule, the less invasive a procedure, the less likely it is to achieve a significant change in the eye pressure or continue to function long-term. Therefore, there is a trade-off between the degree of safety and the effectiveness of the procedure.
Some MIGS procedures (such as the iStent and Hydrus) are reserved for more mild types of glaucoma that are being treated at an early stage. Other MIGS procedures (such as the XEN stent) can be performed on more advanced glaucomas.
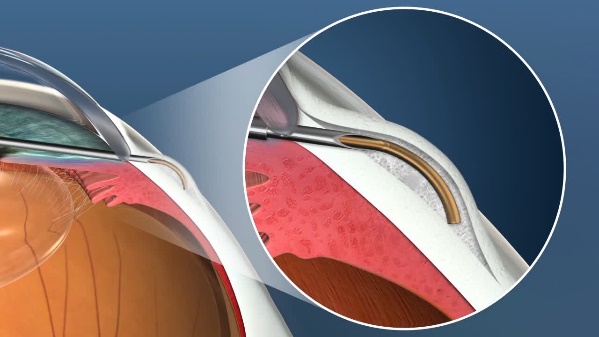
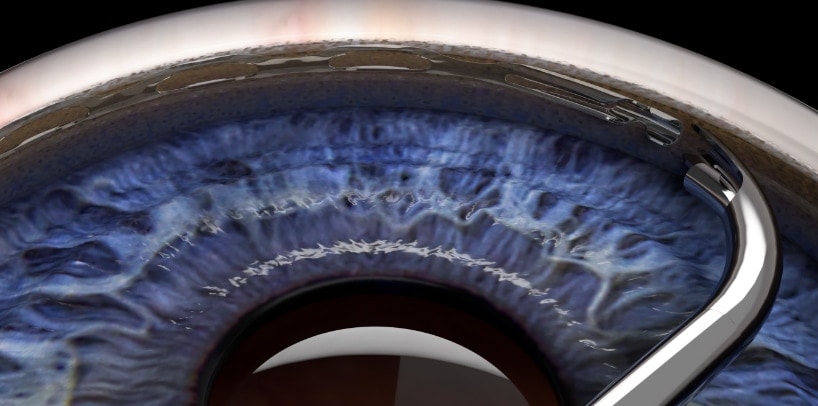
The XEN Gel Microstent is a very small glaucoma implant designed to reduce intraocular pressure in difficult to control glaucoma cases. It is placed using a fine delivery injector. It creates a channel that connects the anterior chamber to the subconjunctival space, allowing aqueous humor to flow into a small reservoir called a bleb. The fluid is then absorbed into the blood stream around the eye. The stent is designed to remain in the eye permanently.
Relative Size of the XEN Stent
Position of the XEN Stent in the Eye
The XEN stent can be placed in the eye alone or combined with cataract surgery. It is placed through a tiny incision in the cornea that is self-sealing. The recovery is rapid, and most patients can resume regular activities the next day.
The XEN is designed to reduce the risk of problems that are sometimes seen with larger, conventional glaucoma implants.
The XEN represents a major advance in glaucoma surgery because it provides the benefits of good reduction in intraocular pressure seen with larger shunts, with the safety profile seen with the smaller MIGS devices.
As with all glaucoma surgeries, the XEN stent may fail to control the eye pressure at some point after the surgery. If this occurs, additional surgery may be required.
The iStent Micro-Bypass glaucoma implant is the smallest of the MIGS devices. It is used for mild or moderate cases of glaucoma where the intraocular pressure is already adequately controlled with topical medications. The iStent can allow the patient to reduce or eliminate glaucoma eye drops.
The iStent is always placed at the time of cataract surgery. The stent is placed in the trabecular meshwork to allow aqueous to flow more freely and leave the eye through its natural pathway. The iStent is delivered with an injector through the same self-sealing corneal incision used for the cataract surgery. Two individual stents are placed during the same procedure, in separate locations of the trabecular meshwork.
Relative Size of the iStent
Position of iStents in the Eye
Because the device is so small, there are fewer risks of problems associated with it compared to the large glaucoma implants. In addition, it does not add any additional recovery time to the cataract surgery. However, its small size limits the amount of IOP lowering that can be achieved, which is why it is reserved for glaucoma that is at an early stage.
The Hydrus Microstent is a glaucoma implant that is part of the MIGS group of devices. It is used for mild or moderate cases of glaucoma where the intraocular pressure is already adequately controlled with topical medications. The Hydrus can allow the patient to reduce or eliminate glaucoma eye drops.
Relative Size of the Hydrus Stent
Placement of the Hydrus in the Eye
The Hydrus is always placed at the time of cataract surgery. The stent is placed in Schlemm’s canal to allow aqueous to more freely and leave the eye through its natural pathway. The Hydrus is placed with a special delivery device through the same self-sealing corneal incision used for the cataract surgery.
Because the device is so small, there are fewer risks of problems associated with it compared to the large glaucoma implants. In addition, it does not add any additional recovery time to the cataract surgery. However, its small size limits the amount of IOP lowering that can be achieved, which is why it is reserved for glaucoma that is at an early stage.
Glaucoma Drainage Implants are a group of procedures that are the conventional incisional surgeries for glaucoma. These surgeries are indicated for more advanced glaucomas, where the disease is more severe, and the eye pressure is much more difficult to control. Glaucoma drainage implants are often used for secondary glaucomas.
Glaucoma drainage implants are larger devices and require more manipulation to implant. The surgeries take longer to perform than the MIGS procedures, and there is a longer recovery period. However, they are necessary in cases where MIGS procedures will not work.
The risks of post-operative problems are increased with glaucoma drainage implant surgeries, in part, because the eye pressures are usually extremely high to start, and the eyes have more complex glaucoma problems.
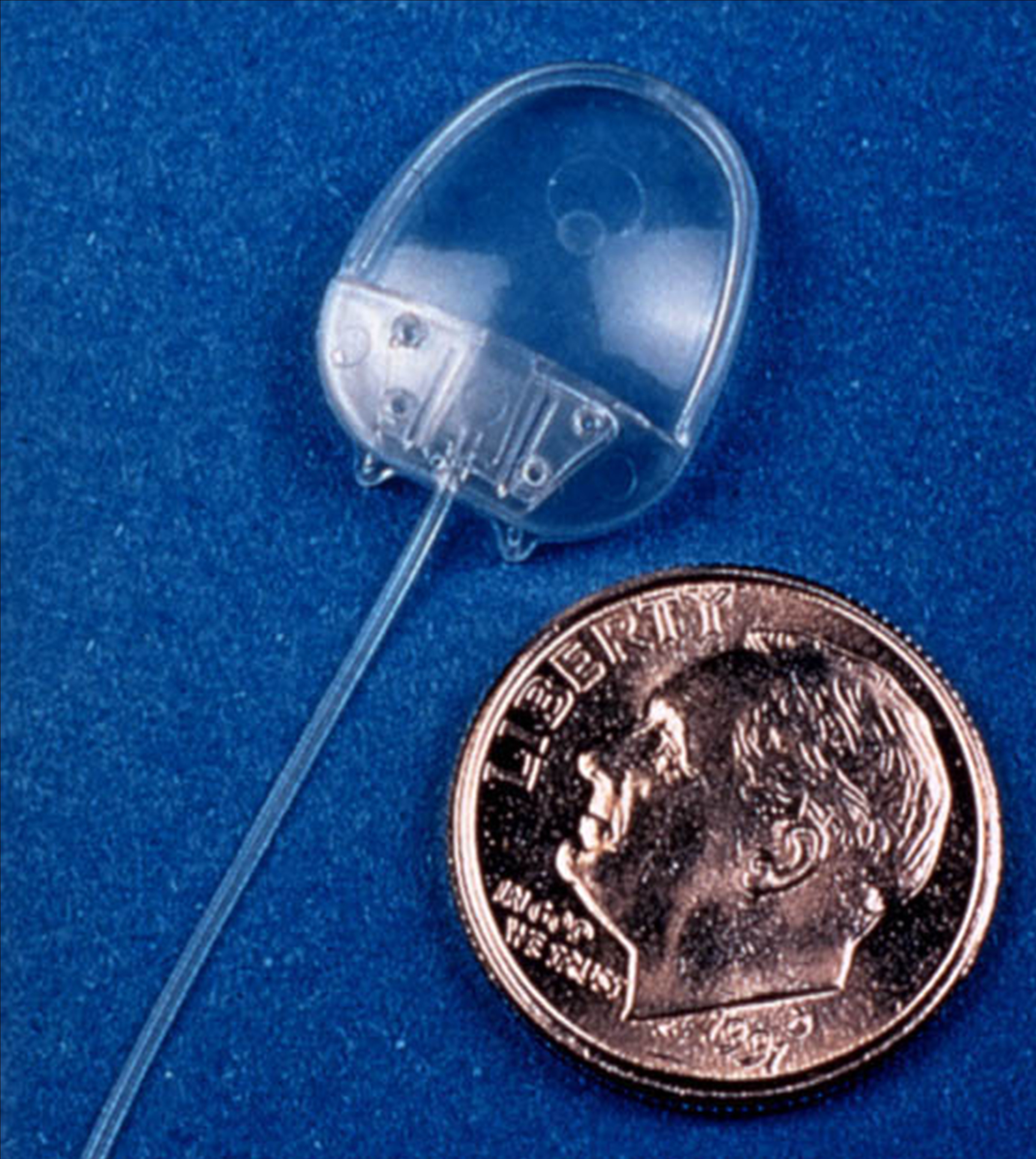
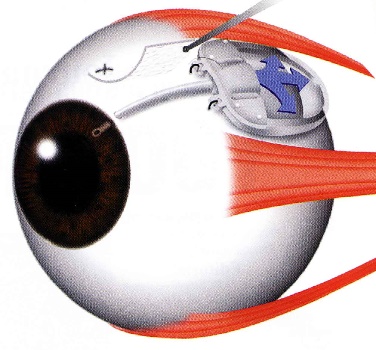
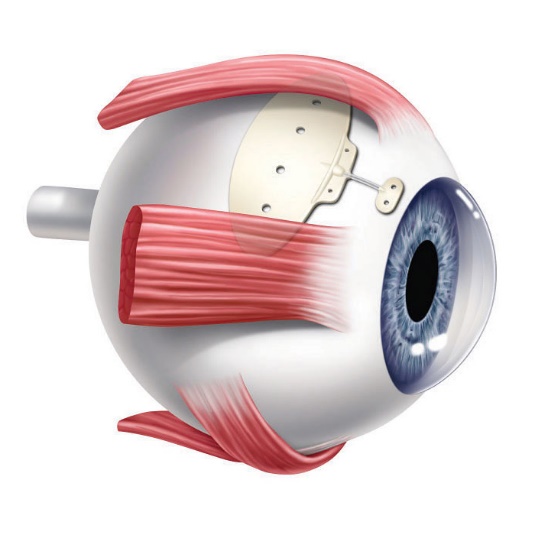
The Ahmed Glaucoma Valve is a commonly used glaucoma implant. The device is composed of plastic and consists of a tube connected to a plate with a valve mechanism. The tube is inserted into the anterior chamber of the eye, and the plate is secured to the eye further back. The tube drains the aqueous fluid out of the eye into a reservoir above the plate. When fully healed, the patient does not feel or see the device in place.
Relative size of the Ahmed Glaucoma Valve
Position of the Ahmed Valve on the Eye
The surgery to place the Ahmed valve usually takes about 45 minutes to perform and is done under local anesthesia. The healing time can take up to a few weeks, but patients can resume most regular activities quickly.
The valve functions to lower the intraocular pressure immediately and is designed to work long-term. Because of the relatively large diameter of the tube, and the position of the plate, the valve is not at high risk for scarring. However, the eye pressure does not always end up as low with the Ahmed valve as with some other glaucoma devices.
The Ahmed valve is preferred when the goal is rapid lowering of intraocular pressure.
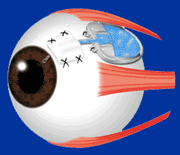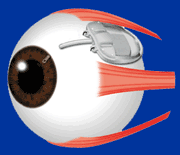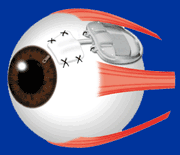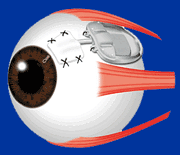
The Baerveldt Drainage Implant is the most reliable of all the glaucoma surgeries. It is very effective at achieving low intraocular pressure and is likely to function long-term. The device is composed of plastic and consists of a tube connected to a large plate, but with no valve mechanism. The tube is inserted into the anterior chamber of the eye, and the plate is secured to the eye further back. The tube drains the aqueous out of the eye into a reservoir above the plate. When fully healed, the patient does not feel or see the device in place.
Baerveldt Drainage Implant
Dr. Tesser performing Baerveldt Implant Surgery
The Baerveldt implant surgery takes longer to perform than other glaucoma surgery, and there is usually a longer healing time, up to several weeks, due to its larger size. There is often a delay in the lowering of the eye pressure because the non-valved tube of the device is intentionally tied off with a suture designed to dissolve very slowly. Because of the relatively large diameter of the tube, and the size and position of the plate, the shunt is not at high risk for scarring, and can achieve low eye pressures. The Baerveldt glaucoma implant is best suited for eyes requiring low final eye pressures, and in situations where the other glaucoma devices are likely to fail.
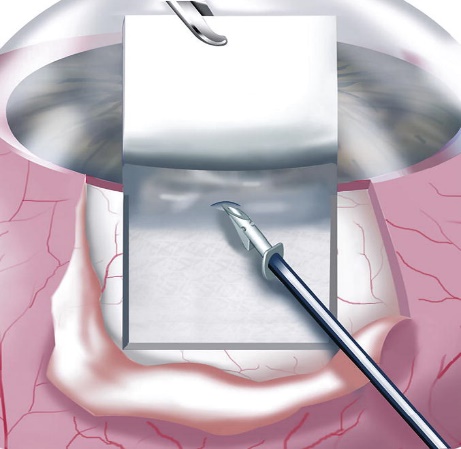
The Ex-Press Mini Shunt is a smaller glaucoma drainage implant device and the surgery, as its names implies, is quicker to perform. The Ex-Press Mini Shunt is a variation of a standard glaucoma operation procedure called a trabeculectomy, often called glaucoma filtration surgery.
The Ex-Press shunt is made of stainless steel. It is a small stent that is placed under a flap of sclera and inserted into the anterior chamber. Aqueous humor fluid flows through the shunt out of the eye into a small reservoir called a bleb. The aqueous is then absorbed by the bleb into the blood circulation of the eye:
Ex-Press Shunt Implantation Under Scleral Flap
Drainage of aqueous humor through the Ex-Press Shunt
The Ex-Press shunt is designed to remain in the eye permanently. It can achieve very low intraocular pressures because the bleb that it creates is often very thin. However, because of its small size it can also stop working due to scar tissue. The Ex-Press Mini Shunt is preferred when the goal of the glaucoma surgery is to achieve the lowest possible pressure.
Cataract and Glaucoma Surgery are often performed together as combined procedures. This is because there is significant overlap between cataracts and glaucoma.
The lens of the eye, because of its position and size, can have significant effects on the fluid flow in the eye, causing the eye pressure to increase. Some glaucoma conditions, such as angle closure glaucoma, are the result of an enlarging cataract.
Some conditions, such as steroid use or diabetes, can cause both cataracts and glaucoma.
Some secondary glaucomas, such as exfoliation glaucoma, are associated with both complex glaucoma and cataracts, each of which more difficult to treat.
As a result, in these and other situations, it is often more efficient to combine the glaucoma and cataract surgeries. The combined surgery can simultaneously achieve both improved vision and a lowering of the eye pressure. In addition, it is more convenient for the patient to have both types of surgery at the same time.
In cases where the glaucoma is mild, and there is a coexisting cataract, combining cataract surgery with a MIGS procedure can reduce or eliminate the number of glaucoma drops that need to be used. In more severe glaucomas, the cataract surgery can be combined with a glaucoma drainage implant.
In certain situations, however, it is best to perform sequential procedures, at different times. This often depends on the relative complexity and severity the cataract or the glaucoma. Either the glaucoma surgery or the cataract surgery can be performed first, followed by the second type of procedure. The time between the procedures can be anywhere from weeks to months, in order to allow the eye to recover form the first procedure.
Glaucoma Surgery is incisional surgery performed on the eye, in an operating room, using an operating microscope. Glaucoma surgery can be performed by itself, or sometimes combined with cataract surgery. There are many different types of glaucoma surgery.
All glaucoma surgeries share in common the purpose of increasing the drainage of aqueous humor fluid from the eye in order to lower the pressure inside the eye.
Glaucoma surgery procedures can be minimally invasive, taking just minutes to perform with rapid recovery, or can be more complex procedures that take longer to perform with longer recovery times. The surgeries are almost always performed as same day, outpatient surgery.
The type of surgery that is best suited for a patient depends on many factors including the type of glaucoma, how high the pressure is, how low the pressure needs to be, how quickly the pressure needs to be lowered, the stage of the glaucoma, and the overall health of the eye.
Minimally Invasive Glaucoma Surgery (MIGS) refers to a group of procedures that are performed relatively quickly with little manipulation to the eye, usually under topical (eye drop) anesthesia, using small incisions and no sutures. The goal of a MIGS procedure is to provide rapid recovery while minimizing the risks associated with conventional glaucoma surgery.
As a general rule, the less invasive a procedure, the less likely it is to achieve a significant change in the eye pressure or continue to function long-term. Therefore, there is a trade-off between the degree of safety and the effectiveness of the procedure.
Some MIGS procedures (such as the iStent and Hydrus) are reserved for more mild types of glaucoma that are being treated at an early stage. Other MIGS procedures (such as the XEN stent) can be performed on more advanced glaucomas.
The XEN Gel Microstent is a very small glaucoma implant designed to reduce intraocular pressure in difficult to control glaucoma cases. It is placed using a fine delivery injector. It creates a channel that connects the anterior chamber to the subconjunctival space, allowing aqueous humor to flow into a small reservoir called a bleb. The fluid is then absorbed into the blood stream around the eye. The stent is designed to remain in the eye permanently.
Relative Size of the XEN Stent
Position of the XEN Stent in the Eye
The XEN stent can be placed in the eye alone or combined with cataract surgery. It is placed through a tiny incision in the cornea that is self-sealing. The recovery is rapid, and most patients can resume regular activities the next day.
The XEN is designed to reduce the risk of problems that are sometimes seen with larger, conventional glaucoma implants.
The XEN represents a major advance in glaucoma surgery because it provides the benefits of good reduction in intraocular pressure seen with larger shunts, with the safety profile seen with the smaller MIGS devices.
As with all glaucoma surgeries, the XEN stent may fail to control the eye pressure at some point after the surgery. If this occurs, additional surgery may be required.
The iStent Micro-Bypass glaucoma implant is the smallest of the MIGS devices. It is used for mild or moderate cases of glaucoma where the intraocular pressure is already adequately controlled with topical medications. The iStent can allow the patient to reduce or eliminate glaucoma eye drops.
The iStent is always placed at the time of cataract surgery. The stent is placed in the trabecular meshwork to allow aqueous to flow more freely and leave the eye through its natural pathway. The iStent is delivered with an injector through the same self-sealing corneal incision used for the cataract surgery. Two individual stents are placed during the same procedure, in separate locations of the trabecular meshwork.
Relative Size of the iStent
Position of iStents in the Eye
Because the device is so small, there are fewer risks of problems associated with it compared to the large glaucoma implants. In addition, it does not add any additional recovery time to the cataract surgery. However, its small size limits the amount of IOP lowering that can be achieved, which is why it is reserved for glaucoma that is at an early stage.
The Hydrus Microstent is a glaucoma implant that is part of the MIGS group of devices. It is used for mild or moderate cases of glaucoma where the intraocular pressure is already adequately controlled with topical medications. The Hydrus can allow the patient to reduce or eliminate glaucoma eye drops.
Relative Size of the Hydrus Stent
Placement of the Hydrus in the Eye
The Hydrus is always placed at the time of cataract surgery. The stent is placed in Schlemm’s canal to allow aqueous to more freely and leave the eye through its natural pathway. The Hydrus is placed with a special delivery device through the same self-sealing corneal incision used for the cataract surgery.
Because the device is so small, there are fewer risks of problems associated with it compared to the large glaucoma implants. In addition, it does not add any additional recovery time to the cataract surgery. However, its small size limits the amount of IOP lowering that can be achieved, which is why it is reserved for glaucoma that is at an early stage.
Glaucoma Drainage Implants are a group of procedures that are the conventional incisional surgeries for glaucoma. These surgeries are indicated for more advanced glaucomas, where the disease is more severe, and the eye pressure is much more difficult to control. Glaucoma drainage implants are often used for secondary glaucomas.
Glaucoma drainage implants are larger devices and require more manipulation to implant. The surgeries take longer to perform than the MIGS procedures, and there is a longer recovery period. However, they are necessary in cases where MIGS procedures will not work.
The risks of post-operative problems are increased with glaucoma drainage implant surgeries, in part, because the eye pressures are usually extremely high to start, and the eyes have more complex glaucoma problems.
The Ahmed Glaucoma Valve is a commonly used glaucoma implant. The device is composed of plastic and consists of a tube connected to a plate with a valve mechanism. The tube is inserted into the anterior chamber of the eye, and the plate is secured to the eye further back. The tube drains the aqueous fluid out of the eye into a reservoir above the plate. When fully healed, the patient does not feel or see the device in place.
Relative size of the Ahmed Glaucoma Valve
Position of the Ahmed Valve on the Eye
The surgery to place the Ahmed valve usually takes about 45 minutes to perform and is done under local anesthesia. The healing time can take up to a few weeks, but patients can resume most regular activities quickly.
The valve functions to lower the intraocular pressure immediately and is designed to work long-term. Because of the relatively large diameter of the tube, and the position of the plate, the valve is not at high risk for scarring. However, the eye pressure does not always end up as low with the Ahmed valve as with some other glaucoma devices.
The Ahmed valve is preferred when the goal is rapid lowering of intraocular pressure.
The Baerveldt Drainage Implant is the most reliable of all the glaucoma surgeries. It is very effective at achieving low intraocular pressure and is likely to function long-term. The device is composed of plastic and consists of a tube connected to a large plate, but with no valve mechanism. The tube is inserted into the anterior chamber of the eye, and the plate is secured to the eye further back. The tube drains the aqueous out of the eye into a reservoir above the plate. When fully healed, the patient does not feel or see the device in place.
Baerveldt Drainage Implant
Dr. Tesser performing Baerveldt Implant Surgery
The Baerveldt implant surgery takes longer to perform than other glaucoma surgery, and there is usually a longer healing time, up to several weeks, due to its larger size. There is often a delay in the lowering of the eye pressure because the non-valved tube of the device is intentionally tied off with a suture designed to dissolve very slowly. Because of the relatively large diameter of the tube, and the size and position of the plate, the shunt is not at high risk for scarring, and can achieve low eye pressures. The Baerveldt glaucoma implant is best suited for eyes requiring low final eye pressures, and in situations where the other glaucoma devices are likely to fail.
The Ex-Press Mini Shunt is a smaller glaucoma drainage implant device and the surgery, as its names implies, is quicker to perform. The Ex-Press Mini Shunt is a variation of a standard glaucoma operation procedure called a trabeculectomy, often called glaucoma filtration surgery.
The Ex-Press shunt is made of stainless steel. It is a small stent that is placed under a flap of sclera and inserted into the anterior chamber. Aqueous humor fluid flows through the shunt out of the eye into a small reservoir called a bleb. The aqueous is then absorbed by the bleb into the blood circulation of the eye:
Ex-Press Shunt Implantation Under Scleral Flap
Drainage of aqueous humor through the Ex-Press Shunt
The Ex-Press shunt is designed to remain in the eye permanently. It can achieve very low intraocular pressures because the bleb that it creates is often very thin. However, because of its small size it can also stop working due to scar tissue. The Ex-Press Mini Shunt is preferred when the goal of the glaucoma surgery is to achieve the lowest possible pressure.
Cataract and Glaucoma Surgery are often performed together as combined procedures. This is because there is significant overlap between cataracts and glaucoma.
The lens of the eye, because of its position and size, can have significant effects on the fluid flow in the eye, causing the eye pressure to increase. Some glaucoma conditions, such as angle closure glaucoma, are the result of an enlarging cataract.
Some conditions, such as steroid use or diabetes, can cause both cataracts and glaucoma.
Some secondary glaucomas, such as exfoliation glaucoma, are associated with both complex glaucoma and cataracts, each of which more difficult to treat.
As a result, in these and other situations, it is often more efficient to combine the glaucoma and cataract surgeries. The combined surgery can simultaneously achieve both improved vision and a lowering of the eye pressure. In addition, it is more convenient for the patient to have both types of surgery at the same time.
In cases where the glaucoma is mild, and there is a coexisting cataract, combining cataract surgery with a MIGS procedure can reduce or eliminate the number of glaucoma drops that need to be used. In more severe glaucomas, the cataract surgery can be combined with a glaucoma drainage implant.
In certain situations, however, it is best to perform sequential procedures, at different times. This often depends on the relative complexity and severity the cataract or the glaucoma. Either the glaucoma surgery or the cataract surgery can be performed first, followed by the second type of procedure. The time between the procedures can be anywhere from weeks to months, in order to allow the eye to recover form the first procedure.
Make an Appointment